Graphing Atom Orbital Calculator Program
Windows XP Professional Download Full Version ISO For [32-64] Bit: Windows XP Professional Download Full Version. It is bootable Windows For 32-bit and 64-bit. Apr 09, 2012 This file is a CD image file for users who wish to create an update CD for Windows XP Service Pack 3, for example for offline installation by administrators. Note: Customers running Microsoft Dynamics Retail Management System (RMS) are advised to install a hotfix for a Microsoft Dynamics RMS issue prior to installing Windows XP. Windows XP ISO Download Free Full Version Window. It is Bootable Windows For Windows XP ISO Download For 32-bit and 64bit. Windows xp full iso indir.
Summary Molecular orbitals editor allows building, analyzing and graphical editing of molecular/Kohn-Sham orbitals diagrams from the results of quantum-chemical calculations Usage Open the output file produced by popular quantum-chemical calculation programs (USGamess, PCGamess, Gaussian, Q-Chem, Spartan) containing molecular orbitals data: Click File-Open menu and select the file containing molecular orbitals data Note to the Gaussian users: type #P pop=full GFInput in Gaussian input files. #P option enables extended printout, pop=full enables printout of all molecular orbitals coefficients needed to calculate density and GFInput option enables printout of basis set data (description of primitives in basis set You will see the molecular orbitals energy levels diagram, you can - click menu File-Add New Diagram and select more files Analysis/Graphical editing of molecular orbitals: To select custom energy range for your diagram use the menu View-Select energy range.

Electron Orbital Calculator Applet. This applet calculates the electron shells of arbitrary elements in quantum number order, which turns out to disagree with. Blog Wrong Cd Key Arma 2 Oa Patch. 1/29/2017 0 Comments n. Graphing Atom Orbital Calculator Program In C#. Chemistry Java Applets. Molecular orbitals editor. By selecting Orbital/Peak Selection tool and clicking on the molecular orbital energy level of interest you will see in AO.
Also use the menu items Edit-Zoom In, Edit- Zoom out or Edit- Show All Another way is to place the mouse to the energy axis then press left button and holding it move up or move down to select the range: In select a tool for analysis/editing, e.g. By selecting and clicking on the molecular orbital energy level of interest you will see in decomposition of the selected molecular orbital into atomic orbitals, Use to examine the contributions only from atomic orbitals of interest; see also,; Graphic design of molecular orbitals diagrams Use tools from (, ) for graphical editing the molecular orbitals diagram. To edit the diagram Title and Foot text double-click it with a mouse left button and start edit text. If the title (foot) is invisible then you may enable it MO options window (click Edit - MO diagram options menu). You can use the wide range of font styles using the Format panel (like you do it in the most rich-text processors): To use it select some text fragment and select in the Format panel the needed style; Note Use the menu item Edit - Undo/Redo (or shortcut Ctrl+Z/Ctrl+Y) to undo/redo the actions. Diagram options You can customize many display parameters of a molecular orbitals diagram e.g. Color, size of MO level; distances between individual diagrams (diagrams corresponding to individual MOs data); background color etc.
To use it click the Edit - MO diagram options menu and in the appeared window set the options: Document Save/Export After working with document you may choose to:. Save it to continue edit it in the future, e-mail it to your colleagues etc. Select File-Save As. Menu then in the file filter select 'Chemissian document file (.orbs)', specify the file name and click Save button. Save the document as a picture - in the file filter select raster.bmp format or vector.emf format; in this case you will not be able to edit the document with Chemissian in the future;. Vector format is preferable because it can be made any size without losing its quality and becoming pixelated. Copy the document as a picture to clipboard and paste it into another editor (e.g.
Word where you are preparing your publication): Select File-Copy image to clipboard menu or use Ctrl+B shortcut.
Yes, there are equations, they are called spherical harmonics. They are given here (look at equations 18 through 33): In my opinion, if you are not interested in the math of QM it isn't worth dealing with them, it would be better to print out a piece of paper with the pictures on it. QM math does look rather difficult to solve and Understand it too for me, but I'm so interested in calculating the probability of finding an electron of a given distance from the nucleus I just thought that maybe for somebody inexperienced as I am would just be given somekind of formula together with a introductory tutorial explaining the physics behind the formula-made easy for me to understand withought all the technical terms. I am just interested in the qualitative understanding behind orbital shapes and calculating the probability of finding electrons at a given distance from the nucleus (if there is a formula(s) for that). That's pretty much the idea behind my post. I want an understanding in simple bunny-easy terms.
LOL i know i sound like an idiot but i just completed Grade 12 and with no knowledge or familiarity of what is harmonics or whatever I deserve an explanation in very simple terms. I remember my chem teacher in class saying, 'the electron dematerializes into radiation and then rematerializes back into matter just like star treck' I was so overwhelmed by that i spent endless days trying to picture how electrons show complimentarity of waves and particles. That's the whole reason why i wanted to learn orbitals and all that neat stuff. If there are any resources available like courses or online tutorials or pretty much ANYTHING out there that can help me understand princples and dynamics of orbitals I'd be filled with delight. One question: Whenever i think about wave-particle duality is it reasonable to picture it like this? A wave packet that has energy concentrated in one fraction of space-time but thats only if picturing a hypothetical 'eye' close-up view.
Now, take the eye away from the 'wave-packet' has to increase the dimension of space-time and the 'wave-packet' pictures so concentrated in space-time that to us now, in the new 'zoomed-out' view of the dimension the wave packet seems like a localized particle but is actually a small little wave concentrate and tightly packed with energy-some sort of energy i do not know what to call it appropriately but some form of energy indeed. Am i correctly picturing wave-particle duality in my sense? Or is there another view which is more correct but translates into the same picture as mine? Is the virial theorem true for atoms and molecules? It says: The total energy (kinetic + potential) of an electron in an atom or a molecule is always one-half its potential energy.
Thus, for example, when an electron is shifted from a 1s to a 2s orbital, its potential energy increases by 3.27 aJ. At the same time the electron slows down and its kinetic energy drops by half this quantity, namely, 1.635 aJ. The net result is that the total energy (kinetic + potential) increases by exactly half the increase in potential energy alone; i.e., it increases by 1.635 aJ. A similar statement can he made for any change inflicted on any electron in any atomic or molecular system. This result is known as the virial theorem. Because of this theorem we can, if we want, ignore the kinetic energy of an electron and concentrate exclusively on its potential energy.
Electron Orbital Calculator
Now that is just wierd. I always thought that atomic orbitals are independent of each other. I dont understand why they partially overlap with the next higher energy orbital(s).
If this truly happens then shouldnt lets say an electron in 2p orbital have an energy similiar to the next 3s orbital? That's considering the case where atomic orbitals overlap.
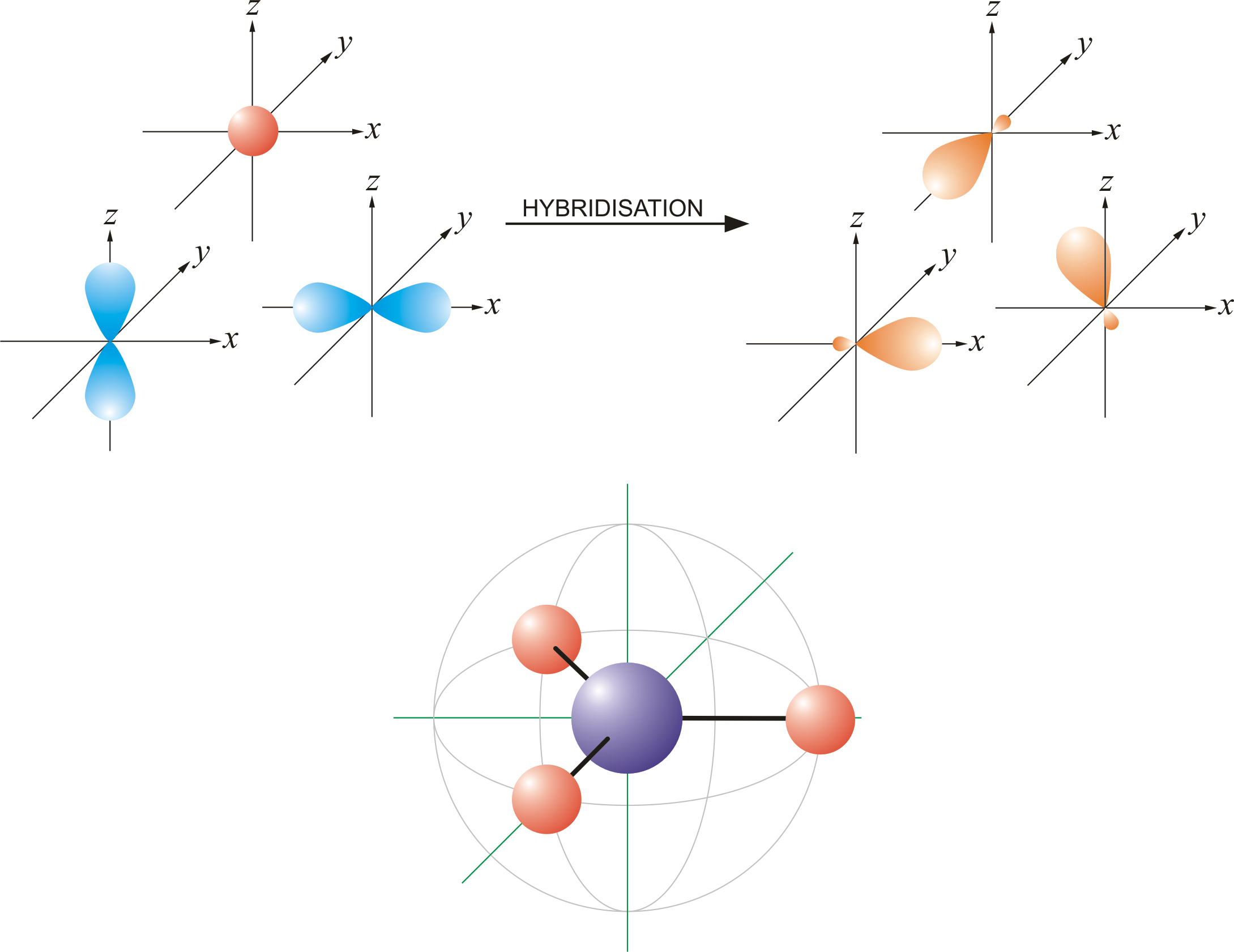
Lithium Atom Orbital
Why do they overlap anyway? In all chemistry textbooks they never show an atom with multiple orbitals illustrated. What would an atom REALLY look like if all the orbitals were filled and a physical model were made in real life considering the overlap as well of orbitals. I know orbitals are non-physical entities but just to give me a general idea? And btw that site is just for hydrogen what about for multi-electron atoms?